|
Summertime is here!! You want to have fun and stay protected. The following list is important for your first aid kit:
1. Adhesive bandages - different sizes 2. Adhesive tape - including waterproof 3. Antibiotic application - use according to directions 4. Antiseptic - use according to directions 5. Eye patch/covering 6. PPE: gloves, mask, goggles, hand sanitizer 7. Roller bandages - different sizes 8. Bandage scissors 9. Tourniquet - manufactured tourniquet to stop bleeding 10.Triangular bandage 11. Needle nose pliers - remove tics 12. Straight edge object - remove bee stinger 13. Cloths, bandanas - to control bleeding or can be used to hold a sling in place These are just a few items that should be in your first aid kit. It may also include sunscreen and bug repellent. Be safe and have fun this summer. Keep a first aid kit in your home, office and car. Stay well & healthy! Johnnita Woods Parker, RN BSN NHDP-BC CPR Life Support Network, LLC www.cprlifesupport.com Resource: American Heart Association Heartsaver First Aid
2 Comments
Learn How to Save a LifeIt's important to spotlight CPR & AED Awareness for all Americans to educate and inform on how lives can be saved if more people learn CPR and how to use an AED.
Each year, more than 350,000 EMS-assessed out-of-hospital cardiac arrests occur in the United States. When a person has a cardiac arrest, survival depends on immediately receiving CPR from someone nearby. According to the American Heart Association, about 90 percent of people who suffer out-of-hospital cardiac arrests die. CPR, especially if performed immediately, can double or triple a cardiac arrest victim’s chance of survival. If you are called on to give CPR in an emergency, you will most likely be trying to save the life of someone you love. The importance is on the willingness of bystanders to act in a cardiac arrest emergency. Did you know about 70 percent of out-of-hospital cardiac arrests happen in homes? If you are called on to give CPR in an emergency, you will most likely be trying to save the life of someone you love. Be the difference for your parent, spouse, child, family-member, friend or co-worker. What if it were you? Take 90 Seconds to learn how to save a life. Are there different types of hyperthermia?
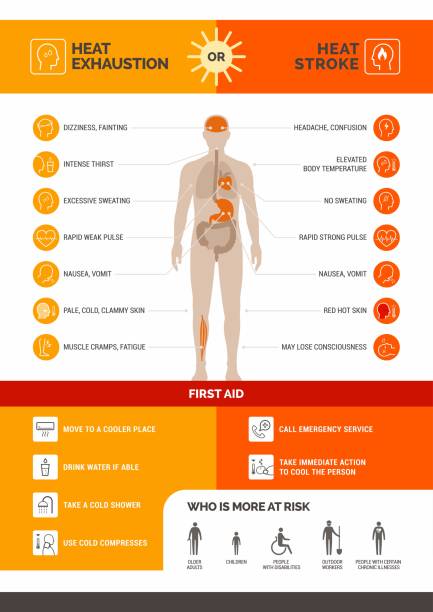
Hyperthermia describes a group of heat illnesses that include (from least to most severe):
Is your medicine cabinet full of expired drugs or medications you no longer use? Your medicine is for you. What’s safe for you might be harmful for someone else. The best way to dispose of your expired, unwanted, or unused medicines is through a drug take back program — or you can do it at home.
Drug Take Back ProgramsThe U.S. Drug Enforcement Administration (DEA) sponsors National Prescription Drug Take Back Day in communities nationwide. Many communities also have their own drug take back programs. Check with your local law enforcement officials to find a location near you or with the DEA to find a DEA-authorized collector in your community. You can also check with your pharmacist. Some pharmacies offer on-site medicine drop-off boxes, mail-back programs, and other ways to help you safely dispose your unused medicines. How to Dispose of Medicines at Home: When a take back option is not easily available, there are two ways to dispose of medicines at home, depending on the drug. Flushing medicines: Because some medicines could be especially harmful to others, they have specific directions to immediately flush them down the sink or toilet when they are no longer needed, and a take-back option is not readily available. How will you know? Check the label or the patient information leaflet with your medicine. Or consult the U.S. Food and Drug Administration’s list of medicines recommended for disposal by flushing when a take back option is not readily available. Remember, don’t flush your medicine unless it is on the flush list. Disposing medicines in household trash: If a take back program is not available, almost all medicines, except those on the FDA flush list (see below), can be thrown into your household trash. These include prescription and over-the-counter (OTC) drugs in pills, liquids, drops, patches, and creams. Follow these steps:
Disposing Fentanyl Patches: The fentanyl patch is an example of a product that contains a powerful opioid medicine that can be dangerous to people it’s not prescribed for. This adhesive patch delivers a strong pain medicine through the skin. Even after a patch is used, a lot of the medicine remains. That’s why the drug comes with instructions to flush used or leftover patches. Disposing Inhaler Products: One environmental concern involves inhalers used by people who have asthma or other breathing problems, such as chronic obstructive pulmonary disease. Read handling instructions on the labeling of inhalers and aerosol products. These products could be dangerous if punctured or thrown into a fire or incinerator. To properly dispose of these products and follow local regulations and laws, contact your trash and recycling facility. Flushing Drugs and the Water Supply:: Some people wonder if it’s okay to flush certain medicines when a take back option is not easily available. There are concerns about the small levels of drugs that may be found in surface water, such as rivers and lakes, and in drinking water supplies. “The main way drug residues enter water systems is by people taking medicines and then naturally passing them through their bodies,” says Raanan Bloom, Ph.D., an environmental assessment expert at the FDA. “Many drugs are not completely absorbed or metabolized by the body and can enter the environment after passing through wastewater treatment plants.” The FDA and the U.S. Environmental Protection Agency take the concerns of flushing certain medicines in the environment seriously. Still, there has been no sign of environmental effects caused by flushing recommended drugs. In fact, the FDA published a paper to assess this concern, finding negligible risk of environmental effects caused by flushing recommended drugs. For more information on what to do when you no longer need your medicines, visit this FDA page.  Infection Control Compliance for AdministratorsBy Katherine West, RN, BSN, MSEd, DICO-C, James Cross, JD - 3.17.2022 The need for a comprehensive exposure control plan and program has been required since late 1991 when the Occupational Safety and Health Administration (OSHA) issued the Bloodborne Pathogen Standard, 29 CFR 1910.1030.1 These regulations laid out the foundation of what components were needed to establish a program for the protection of employees from contracting an occupationally acquired bloodborne infection. Many employers are not aware that there is a companion document to this standard which gives a more in-depth explanation of the regulations and examples. This document, known as a compliance directive, CPL 02-02.069,2 was published in 1995. Several updates and revisions to this document followed; a major update was published in 2001. The OSHA Bloodborne Pathogens Standard was updated in 2001 pursuant to the Needlestick Safety and Prevention Act, Public Law 106-430, passed by the United States Congress in 2000,3 which required OSHA to update the Standard to address needle safe device requirements. By this time, it had been well established in many studies that injuries from sharps were the leading cause of employee exposures in the healthcare setting and that the reduction in these injuries could be prevented by requiring the use of needle safe devices. OSHA also is enforcing Centers for Disease Control and Prevention (CDC) Guidelines for Tuberculosis, using its authority under the general duty clause, Section 5(a), of the Occupational Safety and Health Act of 1970.4 OSHA has been enforcing these guidelines for high-risk workplaces, including fire/EMS, since 1993. The OSHA compliance directive for Tuberculosis is CPL 02-02-078, which was issued in 2005.5,6 Many departments (and even medical facilities) have missed the fact that OSHA has been enforcing CDC Guidelines and Recommendations for many years via the general duty clause. Administrators and human resource managers often state incorrectly that CDC publishes guidelines and recommendations, which are not laws or requirements, so we do not need to follow them. This statement is far from accurate to say the least. OSHA can issue citations and fines for non-compliance with CDC guidelines and recommendations. Yes, the CDC documents say guidelines/recommendations, but the fact that OSHA is enforcing them essentially makes them OSHA regulations.6 There is also not a clear understanding that fire/EMS personnel are clearly defined as “healthcare personnel” by OSHA and the CDC. The definition states all paid and unpaid persons, so volunteers are clearly addressed. Because of this, many departments are not in compliance with CDC guidelines which are OSHA-enforced – such as requirements for immunizations/vaccinations, work restrictions when employees are ill, and several others.7 In addition to OSHA regulations and enforcement, the Ryan White CARE Act, Public Law 101-381, was passed in 1990. This law requires that every emergency response employer in the country have a “designated officer” to act as a liaison between an exposed employee and medical facilities to which source patients are transported. The position of the designated infection control officer (DICO) in departments became common following enactment of the Ryan White Act. This act creates significant rights and responsibilities for emergency response employers and medical facilities. After employees report an exposure to blood or other potentially infectious material to the DICO, the DICO is responsible for verifying that there was in fact in exposure. After such confirmation, the DICO is responsible for contacting the appropriate personnel at the medical facility to which the source patient was transported and to request the disease status of the patient. Disclosure of a patient’s HIV status is governed by state law, so knowledge of the legal requirements for consent in the state in which business is being conducted is needed. For airborne and droplet transmitted diseases, medical facilities are required by the law to notify the DICO of the transporting department when the facility determines the department had transported a patient suspected of or diagnosed with a disease that was on the list developed by the CDC.8,9,10 The existence of the Ryan White Act, and similar requirements under OSHA regulations and enforcement, has created the need for a trained DICO in each department to ensure employees are receiving appropriate post-exposure medical follow-up and that the department is complying fully with its legal requirements. Meeting these responsibilities requires not only a trained DICO but also support from department administration when problems arise. In addition to the laws mentioned thus far, there is a need for all departments to have confidentiality policies in place. There is potential legal liability for the inappropriate disclosure of confidential information of patients and department employees. Although there is a right to know the disease status of patients following employee exposures, the Health Insurance Portability and Accountability Act (HIPAA) Privacy Regulations must be taken into account as well. Discussion of a patient’s identity and their disease status outside of the context of providing post-exposure medical follow-up is a HIPAA violation and this must be addressed in departmental policy. It should be clear at this point that there is a need for many key players in the process to have a comprehensive department exposure control program. More is needed than just an exposure control plan. Let us discuss who the key players need to be to develop a comprehensive exposure control program and keep up with needed updates and added responsibilities. Key players are: the department chief and administration; legal representative(s); the DICO; medical facility representative(s); medical examiner(s); risk management specialist(s); and human resources. Department chiefs needs to be aware of the applicable laws and the policies and procedures that need to be in place for the department to be compliant. The chief also has a vital role in selecting the individual to be the DICO for the department. This person should be interested in serving in this role, self-motivated, and accepted by the members of the department. Training for this role is essential, although the necessary training is not specified in the Ryan White Act or the Bloodborne Pathogens Standard. Administration needs to fully support the role of the DICO and assist with a budget for this position to cover post exposure costs, vaccines, and immunizations. As this program is designed to ensure that proper post-exposure medical treatment and counseling is given to an exposed employee, careful selection of the physician to deliver post-exposure care and counseling is needed. OSHA is noticeably clear that the department holds the responsibility to ensure that proper care and counseling is afforded an exposed employee. The physician is only acting as an agent on behalf of the department. A key player in a successful comprehensive exposure control program is the DICO. This individual is the key to ensuring that proper care and counseling is afforded each exposed employee. The DICO is to set up a communication system between the medical facility and the patient that is the source of the exposure. Awareness of the state HIV testing law for their state is especially important. It is also important the DICO assists in the source patient receiving rapid testing when an exposure has occurred. The source patient test results are to be given directly to the DICO, who will then review them with the exposed employee. The Ryan White Act is clear that this is to be the process. The medical facility is not to give the results to the employee. The DICO makes the first determination as to whether a reported event is or is not an exposure. If not an exposure, no medical follow up is needed or recommended. This process can be an opportunity for a DICO to conduct some one-on-one education as to why an event is not an exposure. The DICO making this determination benefits administration by not generating unneeded costs. This is an example of the balance the DICO performs between being an advocate for the employee as well as for the department. If an event is determined to be an exposure, then the DICO acts to ensure that proper care is given based on the needs for each employee. The needs for care rendered may differ based on the status of the employee regarding past disease history and vaccination status. Complete documentation of each exposure event is essential for recordkeeping. The DICO needs to maintain records in addition to the medical care provider. This is important for risk management and liability reduction. This process also benefits both the exposed employee and the department. The DICO needs also to ensure that department policies and procedures as well and the exposure control plan are updated and reflective of the current evidence-based practice. Medical facilities also play a key role in having an effective exposure control program. They need to be aware of laws such as the Ryan White Act, which differ from the way medical facilities follow exposures. Medical facilities also need to be aware that OSHA is enforcing CDC guidelines and recommendations and that these are to be followed as laws. This would include contracted departments of a medical facility such as the laboratory and the emergency department. Medical facilities fall under Federal OSHA and need to comply. The key responsibility of the medical facility is to conduct proper testing on the source patient, and this is to be rapid testing as stated in the CDC guidelines. EMS is not to be involved in source patient testing, as stated by the CDC. Legal and risk-management representatives for the department need to be involved and play a significant role in establishing a program that meets all legal requirements. The human resource department plays a role in processing of worker’s compensation but should not be involved in post exposure determination and/or policies. Exposure records are kept by the DICO as they are a confidential medical record. The DICO will share necessary post-exposure information with the workers’ compensation processor. In the case of an exposure involving a deceased person, the medical examiner or coroner holds the responsibility for testing the deceased person. This is a clear requirement in the Ryan White Act; however, this is not a well-known responsibility and the DICO will need to make this requirement known to the medical examiner or coroner. In summary, it is clear that there are many important players in the success of a compliant exposure control program. Compliant programs are cost effect and assist with liability reduction. References
https://www.jems.com/training/jems-con-2022-preview-administrators-course-for-infection-control-compliance/?utm_medium=email&utm_source=jems_now_newsletter&utm_campaign=2022-04-19 |